|
DEXA: Radiation Safety Scintillation Counters |
|
DEXA: Radiation Safety Scintillation Counters |
Intro || Types || Production || Interaction || Attenuation || Biological Effects || Gas Detectors || Scintillation Counters || Protection Principles || Units || Practical || DEXA Theory || Dosimetry || References
This page contains the following sections:
Fluorescent materials e.g.
|
Material |
Radiation |
Comment |
|
Anthracene |
Beta |
- |
|
ZnS(Ag) |
Alpha |
powder |
|
NaI(Tl) |
Gamma |
crystal |
|
CsI(Na) |
X |
crystal |
|
p-terphenyl in toluene |
Gamma |
liquid |
|
p-terphenyl in polystyrene |
Gamma |
plastic |



Let
m: no. of light photons produced in crystal
k: optical efficiency of crystal
l: quantum efficiency of photocathode
n: no. of dynodes
R: dynode multiplication factor
Therefore, the charge collected at the anode
Q = m k l Rn e
e.g. for a 0.1 MeV gamma-ray, typically =>
m = 1,000
k = 0.5
l = 0.15
n = 10
R = 4.5
Therefore, Q = 41 x 10-12 C
=> very sensitive amplifier needed
Voltage pulse measured across RL
is proportional to
the energy deposited in the crystal by the radiation.
These voltages pulses range in amplitude depending on how the radiation interacted with the crystal
i.e. the pulses form a spectrum whose shape depends on the interaction mechanisms involved
e.g. for medium-energy gamma-rays used in in-vivo nuclear medicine:

Allows spectrum to be acquired.
passes voltage pulses > than its setting [ULD]
passes voltage pulses < than its setting [LLD]
=> variable width window which can be placed anywhere along the spectrum,
or used to scan the spectrum
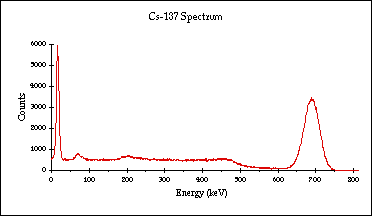
e.g. to detect the photopeak only => ideal gamma-ray spectrum:

Energy of the recoil elctron for 180 degree Compton scattering
=> maximum energy loss due to single Compton effect
Multiple Compton scattering
Detection of gamma-rays that have been scattered in the direction of the detector after undergoing 180 degree Compton scattering outside the detector


Use the selection box to view photos of scintillation detectors
Thermoluminescence
Principle
absorbed radiation energy raises electrons of the detector material to metastable states. They remain in these excited states until the detector is heated to a temperature sufficient to cause them to return to their ground state Ñ and this process gives rise to the emission of light.
The amount of light emitted is directly proportional to the absorbed radiant energy and thus to the absorbed dose.
Common TL Materials
LiF: Mg
LiF: Ti
CaF2: Dy
CaF2: Mn
CaSO4: Dy [used by ARL]
Li2B4O7: Mn
The material is packaged as a pellet or as a crystalline chip enclosed in a holder.
The holder is worn for a period, the TL material is subsequently heated and the light emission measured by a PMT.
Intro || Types || Production || Interaction || Attenuation || Biological Effects || Gas Detectors || Scintillation Counters || Protection Principles || Units || Practical || DEXA Theory || Dosimetry || References
Copyright © Kieran Maher
Last updated: 14 Feb '97